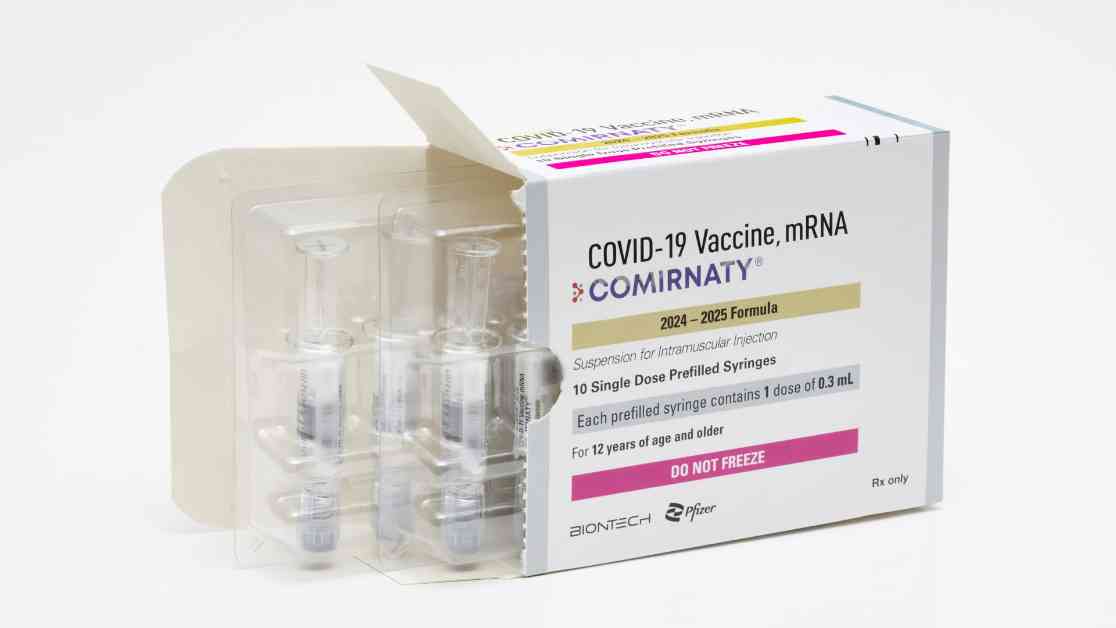
The Food and Drug Administration recently announced the approval of updated Covid vaccines from Pfizer and Moderna, aiming to combat the ongoing surge of the virus in the United States. These new shots are designed to target a strain known as KP.2, which is a descendant of the highly contagious omicron subvariant JN.1 that has been circulating widely in the country since earlier this year.
Updates to the vaccines have become necessary due to the evolving nature of the virus and the emergence of new variants that can potentially evade immunity from previous vaccinations or infections. The FDA’s approval of the updated Pfizer and Moderna vaccines comes at a critical time as the nation grapples with a significant spike in Covid cases during the summer months.
### New Strain, New Response
The dominant Covid strain in May, KP.2, now only accounts for approximately 3% of all cases in the U.S. as of the latest data from the Centers for Disease Control and Prevention. However, Pfizer and Moderna have emphasized that their updated vaccines can generate stronger immune responses against other circulating subvariants of JN.1, such as KP.3 and LB.1, compared to last year’s shots that targeted the omicron strain XBB.1.5.
Dr. Peter Marks, the director of the FDA’s Center for Biologics Evaluation and Research, highlighted the importance of updating Covid vaccines to ensure better protection against current variants. Given the waning immunity in the population from previous exposure to the virus and prior vaccination, it is strongly recommended that eligible individuals consider receiving the updated Covid-19 vaccine.
### Rollout and Availability
Pfizer and Moderna have wasted no time in making their updated vaccines available to the public. Pfizer has already begun shipping its new shot and expects it to be accessible in pharmacies, hospitals, and clinics across the country in the coming days. Moderna also anticipates that its updated vaccine will be available within a similar timeframe.
Moderna CEO Stephane Bancel emphasized the importance of staying up to date with Covid vaccinations to protect against severe illness. Encouraging individuals to speak with their healthcare providers about receiving the updated vaccine alongside their flu shot this fall, Bancel emphasized the role of vaccination in preventing the spread of the virus.
### Federal Recommendations and Response
In June, the CDC recommended that everyone over the age of 6 months receive an updated Covid vaccine and flu shot this year. While the new shots from Pfizer and Moderna are specifically approved for individuals aged 12 and older, they are also authorized for emergency use in children aged 6 months through 11 years.
As the nation faces a resurgence of the virus, with a high level of Covid detected in wastewater across almost every state, the timely approval of updated vaccines offers a glimmer of hope. Despite rising Covid test positivity rates and hospitalizations, federal health officials remain optimistic that the new shots will contribute to curbing the spread of the virus.
### Novavax’s Role and Response
Novavax, a biotech company that had initially targeted the JN.1 strain with its vaccine, has been urged by the FDA to shift focus to the KP.2 variant. While the company’s vaccine provides protection against descendants of JN.1, including KP.2.3, KP.3, KP.3.1.1, and LB.1, the FDA has yet to clear Novavax’s jab for authorization.
Novavax remains optimistic about the potential authorization of its shot in time for peak vaccination season in the U.S. The company’s protein-based vaccines, unlike the mRNA technology used by Pfizer and Moderna, cannot be quickly updated to target new strains of the virus. However, Novavax is working closely with the FDA to ensure the timely approval of its vaccine.
### Public Perception and Response
Despite the availability of updated vaccines and federal recommendations for their administration, the uptake of booster shots among the American population remains a point of concern. Only around 22.5% of U.S. adults received the latest round of shots that were rolled out last fall, according to CDC data.
A November survey from the Kaiser Family Foundation revealed that many individuals who had received previous rounds of Covid shots cited a lack of concern about the virus or being too busy as reasons for not getting the latest booster. With ongoing efforts to educate the public about the importance of vaccination, it remains to be seen how many Americans will opt to receive the updated Covid shots in the coming months.
### Conclusion
The FDA’s approval of updated Covid vaccines from Pfizer and Moderna marks a critical step in the ongoing battle against the virus. With new variants continuing to emerge and immunity from previous vaccinations waning, the availability of updated vaccines offers a ray of hope in the fight against Covid.
As the nation navigates a summer surge in cases, the timely rollout of the new shots provides an opportunity for individuals to bolster their immunity and protect against severe illness. With continued efforts to educate the public and make vaccines accessible, the path towards ending the pandemic remains within reach.