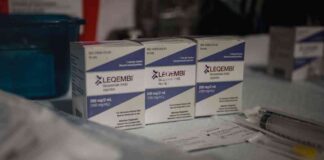
Gilead Sciences, a pharmaceutical company known for its HIV medications, recently uncovered a dangerous drug-counterfeiting operation in New York City. The scheme involved tampering with Gilead’s prescription bottles and improperly reselling the medications to unsuspecting patients. The mastermind behind this operation was identified as Peter Khaim, a twice-convicted medical fraudster. According to court documents unsealed this month, Khaim was described as one of the most brazen and largest manufacturers and sellers of counterfeit Gilead medications in the country.
Gilead Sciences filed a lawsuit against Khaim and two New York City pharmacies, 71st RX and Best Scripts, both located in Queens. The lawsuit also named others connected to the counterfeiting scheme. Gilead alleged that Khaim controlled the two pharmacies and was responsible for manufacturing and trafficking counterfeit Gilead-branded HIV medications to pharmacies and patients in New York and New Jersey. This put the health and safety of countless patients at risk.
The counterfeiters used authentic Gilead prescription bottles but tampered with the medication or associated documentation. The bottles were often emptied, refilled with the wrong medication, and resealed using a different material than Gilead’s authentic tamper-evident seals. The counterfeit bottles were then sold with counterfeit patient information documents, caps, and pedigrees or invoices. The majority of the seized Gilead HIV medications were Biktarvy and Descovy.
Victims of this counterfeiting operation included patients living with HIV who were convinced to give up their prescribed medication and unknowing patients who received counterfeit medications from their neighborhood pharmacies. In July, Gilead attorneys, private investigators, and deputies from the New York City Sheriff’s Office conducted seizures at the two pharmacies and Khaim’s home, confiscating over $750,000 worth of suspected counterfeit medication.
This is not the first time Gilead has taken legal action against Khaim for his involvement in counterfeit HIV medications. In 2021, Gilead sued Khaim and obtained an injunction prohibiting him from selling Gilead-branded products. Despite the injunction, Khaim continued to oversee a counterfeiting operation from the two Queens pharmacies, as revealed in the latest complaint.
In addition to facing civil complaints from Gilead, Khaim has a history of criminal schemes. He received a 96-month prison sentence for a medical fraud case and a 15-year sentence for a separate insurance fraud scheme. Despite wearing a court-ordered GPS ankle monitor, Khaim operated the pharmacies and sold counterfeit medication.
Gilead emphasized its commitment to patient safety and collaboration with regulatory agencies and law enforcement to dismantle counterfeiting networks, deter fraudsters, and prevent illegal pharmaceutical distribution. The company continues to work closely with the FDA, OIG, FBI, and prosecutors to combat counterfeit drugs and protect patients from harm.
Counterfeiting drugs is a serious issue that poses significant risks to public health and safety. In many cases, counterfeiters obtain medications from patients who sell them for cash. The labels are removed using lighter fluid, and the bottles are resealed and dispensed to unsuspecting patients. Lighter fluid was found at the pharmacies during the seizures in the case against Khaim, indicating the use of this method in the counterfeiting operation.
The illicit trade of counterfeit drugs not only harms patients but also undermines the integrity of the pharmaceutical industry. Gilead’s efforts to combat counterfeit medications reflect the company’s dedication to upholding high standards of quality and ensuring that patients receive genuine, safe medications.
Impact on Patients
The discovery of the drug-counterfeiting operation in New York City has had a significant impact on patients, particularly those living with HIV. Patients rely on medications like Biktarvy and Descovy to manage their condition and maintain their health. The counterfeit medications distributed by the pharmacies operated by Khaim posed a serious threat to the well-being of these individuals.
Patients who unknowingly received counterfeit medications may have experienced adverse effects due to taking the wrong medication or incorrect dosages. The tampering with the prescription bottles and associated documentation made it difficult for patients to distinguish between genuine and counterfeit medications. This deception put patients at risk of serious health complications and compromised their treatment plans.
The emotional toll of discovering that they had been given counterfeit medications can also be devastating for patients. Trust in their healthcare providers and pharmacies is essential for patients to feel confident in their treatment and medication regimen. The betrayal of this trust by counterfeiters like Khaim can have long-lasting effects on patients’ willingness to seek medical care and adhere to their prescribed medications.
Regulatory Response
The discovery of the drug-counterfeiting operation in New York City prompted a swift response from regulatory agencies and law enforcement. Gilead’s collaboration with the FDA, OIG, FBI, and prosecutors was instrumental in identifying and dismantling the counterfeit drug network. The seizures conducted at the pharmacies and Khaim’s home in July resulted in the confiscation of a substantial amount of suspected counterfeit medication.
Regulatory agencies play a crucial role in monitoring the pharmaceutical supply chain and ensuring the safety and efficacy of medications. The FDA’s oversight of drug manufacturing, distribution, and labeling is essential to preventing counterfeit drugs from reaching patients. By working closely with pharmaceutical companies like Gilead and law enforcement agencies, regulatory bodies can effectively combat the illegal trade of counterfeit medications and protect public health.
The case against Khaim and the two pharmacies in Queens underscores the importance of robust regulatory enforcement measures to safeguard patients from counterfeit drugs. The collaboration between Gilead, regulatory agencies, and law enforcement agencies demonstrates a united effort to hold counterfeiters accountable and prevent them from endangering patients’ lives.
Public Awareness and Education
The prevalence of counterfeit drugs in the pharmaceutical market highlights the need for increased public awareness and education on the risks associated with purchasing medications from unauthorized sources. Patients must be vigilant when obtaining their medications and ensure that they are purchasing them from reputable pharmacies and healthcare providers.
Pharmacies and healthcare facilities can also play a role in educating patients about the dangers of counterfeit medications and providing guidance on how to identify genuine prescription drugs. By raising awareness about the signs of counterfeit drugs and encouraging patients to report any suspicious activity, healthcare professionals can help prevent patients from falling victim to counterfeiters like Khaim.
Public awareness campaigns and educational initiatives can help empower patients to make informed decisions about their healthcare and medication choices. By arming patients with knowledge about the risks of counterfeit drugs and the importance of verifying the authenticity of their medications, healthcare providers can help protect patients from harm and ensure that they receive safe and effective treatment.
In conclusion, the illegal drug-counterfeiting operation at the NYC pharmacies operated by Peter Khaim is a stark reminder of the dangers posed by counterfeit medications. Gilead Sciences’ lawsuit against Khaim and the pharmacies involved highlights the company’s commitment to protecting patients from counterfeit drugs and ensuring the integrity of the pharmaceutical supply chain. By working collaboratively with regulatory agencies, law enforcement, and healthcare providers, Gilead is taking proactive steps to combat counterfeit medications and safeguard public health. Patients must remain vigilant and informed about the risks of counterfeit drugs to protect themselves from harm and ensure they receive genuine, safe medications for their healthcare needs.